Research focus
The intestinal epithelium is one of the major barriers for blocking outside food-borne pathogens infection into human body. However, several intracellular pathogens such as Salmonella, Shigella, enteroinvasive E. coli (EIEC), and Listeria have been found which can induce internalization by host cells, even penetrate the monolayer epithelium to the submucosal layer, and cause systemic infection by dissemination via the bloodstream and lymphatic fluid. So, the intracellular proliferation of these pathogens within host cells is an important process for their pathogenesis. So far, some cytosolic nutrients such as glutamine and glucose inside host cytosol have been demonstrated which can be utilized by the intracellular pathogens as the energy source. In advance, the analysis by mutagenesis in the pathogens also revealed that carbon metabolism-related enzymes can control their virulence and cytosolic proliferation. According to these evidences, the switching of intracellular metabolism between host cells and their infected cells is a notable issue for studying the pathogenicity of these human pathogens.
The aim of our project is to investigate metabolic crosstalk between intracellular pathogens and their host using the unique possibilities of hyperpolarized tracers. Our preliminary goal is to establish a culture model of gut epithelium and to generate the metabolic fingerprints in the pathogens, the non-infected host cells, and the infected cells by NMR analysis. We suppose that this fingerprint profiles can make an outline of the infectious process. In addition, due to the limitation of the sensitivity in the NMR spectrum, the Stable Isotope Resolved Analysis with Hyperpolarized NMR (SIRAH-NMR) is a new strategy for enhancement of NMR signals and high contrast to noise ratio. Our second goal is to use the SIRAH-NMR to gather the information on the metabolic pathway when the intracellular pathogen adapts to the intracellular lifestyle of the infected host cell. We expect that our studies can provide an alternative field for studying host-pathogen interaction and also for applying to control bacterial infection in the future.
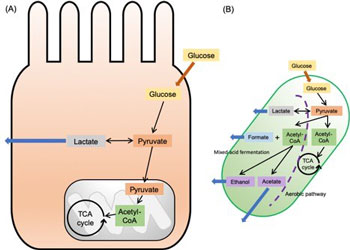
Figure 1. The general glucose metabolic pathways in (A) intestinal epithelial cell and (B) bacterial cell.
Scientific output
Find Ke-Chuan's publications at DTU's online research database ORBIT.
Funding
The project is funded by Danish National Research Foundation as part of the HYPERMAG Center of Excellence (DNRF124).
Project Period
September 2018 - August 2020